First cases of infection from diabetes medication in India
- Posted By
10Pointer
- Categories
Science & Technology
- Published
5th Apr, 2022
-
Context
After the U.S. and Canada, India has reported the first case of Fournier’s Gangrene from Type 2 diabetes patients using sodium-glucose cotransporter-2 (SGLT2) inhibitors.
What is Fournier’s Gangrene?
- Fournier’s gangrene is also called necrotizing fasciitis (flesh-eating disease).
- It is a serious bacterial infection that affects the genitals and areas around the genitals.
- The infection commonly occurs in older men, but it can also occur in women and children.
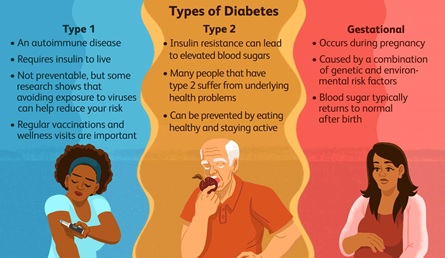
Diabetes
- Diabetes is a chronic, metabolic disease characterized by elevated levels of blood glucose (or blood sugar), which leads over time to serious damage to the heart, blood vessels, eyes, kidneys and nerves.
- The most common is type-2 diabetes, usually in adults, which occurs when the body becomes resistant to insulin or doesn’t make enough insulin.
|
Relation between Fournier’s Gangrene and Diabetes medication
- Sodium-glucose cotransporter-2(SGLT2) inhibitors (a class of Type 2 diabetes medication) are recommended as preferred add-on oral antidiabetic drugs (OADs) after metformin.
- This is preferred for Type-2 diabetes mellitus (T2DM) patients with cardiovascular disease, heart failure (HF) and chronic kidney disease (CKD).
- However, several diabetic patients taking the SGLT2 inhibitor drugs have been infected by Fournier’s Gangrene.
Steps taken by the Government
- Central Drugs Standard Control Organization (CDSCO) has requested all State Drug Controllers to direct the manufacturers of SGLT2 inhibitor class drugs, named Canagliflozin, Dapagliflozin and Empagliflozin under their jurisdiction to include warnings in the package.
About CDSCO
- The Central Drugs Standard Control Organisation (CDSCO) under the Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India is the National Regulatory Authority (NRA) of India.
- Under the Drugs and Cosmetics Act, CDSCO is responsible for approval of New Drugs, Conduct of Clinical Trials, laying down the standards for Drugs, control over the quality of imported Drugs in the country and coordination of the activities of State Drug Control Organizations by providing expert advice with a view to bring about the uniformity in the enforcement of the Drugs and Cosmetics Act.
- CDSCO along with state regulators, is jointly responsible for grant of licenses of certain specialized categories of critical Drugs such as blood and blood products, I. V. Fluids, Vaccine and Sera.
|